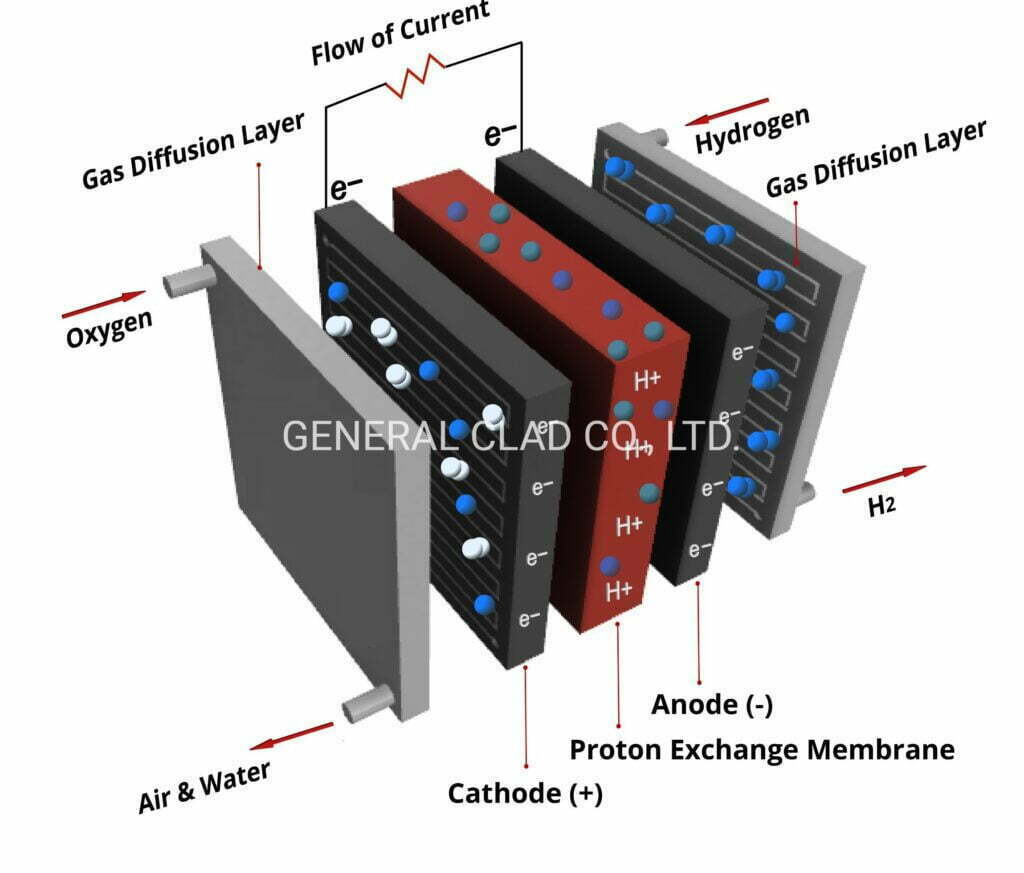
At the present time, in the PEMEC, titanium felt liquid/gas diffusion layer (LGDL) is located between the catalyst layer (CL) and current collecting layer with flow field. As for the purpose of the Titanium felt LGDL, is to transport electrons, heat, and reactants/products to and from the CL with minimal current, thermal, and fluidic losses.
The titanium felt liquid/gas diffusion layer (LGDL) is located between the catalyst layer (CL) and the current distributor, which also acts as the flow field.
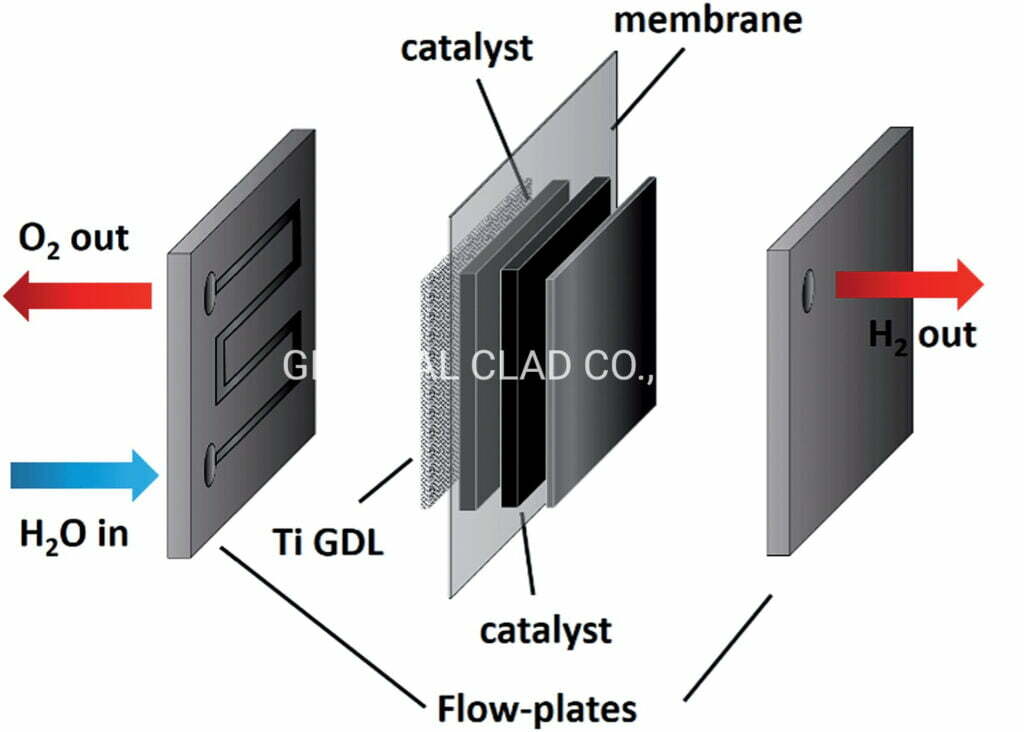
Due to the high voltage and high oxidative environment of the anode electrode, Carbon based materials, typically used in LGDL of PEMFCs. Such as graphite bipolar plates, carbon paper, and carbon cloth. But, they are unsuitable in the anode of LGDL, due to the high anodic potential that occurs during electrolysis operation.
Consequently,titanium felt creates a highly oxidative environment, which corrodes the carbon and results in poor interfacial contacts. Then, the efficiency of the PEM fuel cell will decrease.
In order to resolve this issue, materials with a high corrosion resistance must be used. Titanium felt has received considerable attention as a highly promising material for a PEMEC due to its high corrosion resistance at high positive over potential, even in highly acidic and humid conditions.
In addition, titanium fiber felt provides high thermal/electrical conductivities and excellent mechanical properties. Therefore, titanium is considered to be one of the best materials of making titanium felt LGDL used in PEM fuel cell for hydrogen production.
References(for titanium felt)
1.Ayers, K. E., Dalton, L. T., and Anderson, E. B. “Efficient Generation of High Energy Density Fuel from Water,” 2012, pp. 27-38.
2.Carmo, M., Fritz, D. L., Merge, J., and Stolten, D. “A comprehensive review on PEM water electrolysis,” International Journal of Hydrogen Energy Vol. 38, No. 12, 2013, pp. 4901-4934.
3.Zhang, H. C., Lin, G. X., and Chen, J. C. “Evaluation and calculation on the efficiency of a water electrolysis system for hydrogen production,” International Journal of Hydrogen Energy Vol. 35, No. 20, 2010, pp. 10851-10858.