Green hydrogen (H2) derived from renewable energy sources is the carbon-free fuel of the future. The most popular method of preparing green hydrogen is electrolysis. Water is broken down into oxygen and hydrogen through an electrochemical cell.
This is a simple reaction that yields high-quality hydrogen with zero carbon emissions. Despite these advantages, electrochemical water decomposition has yet to gain significant traction on a commercial scale. This is due to the low conductivity of the active (oxygen) hydroxide catalysts produced in situ during the electrochemical process. This in turn leads to limited catalytic activity. This hinders the hydrogen and oxygen precipitation reactions in the cell.
Poor electrical properties of hydroxides
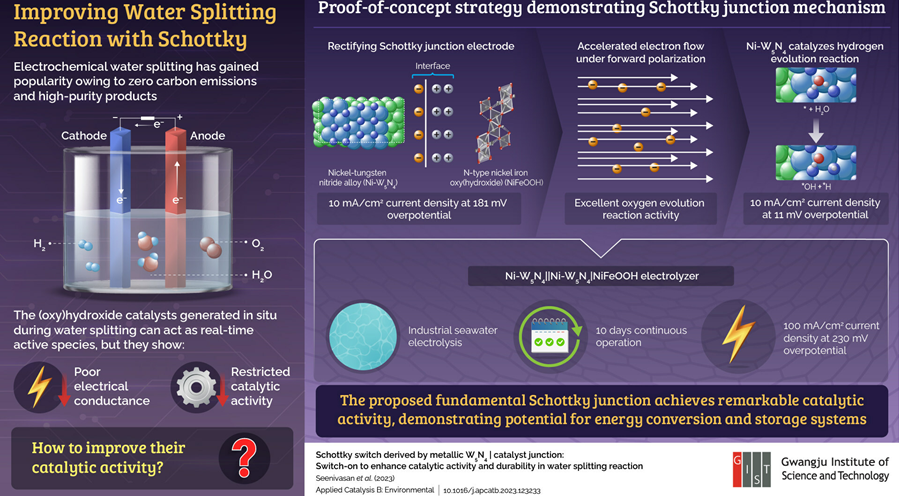
Poor electrical properties of (hydroxy)hydroxides have long been a challenge in achieving efficient water decomposition. Now, a team of researchers led by Junhyeok Seo, an associate professor in the Department of Chemistry at the Gwangju Institute of Science and Technology. It has found a solution to this problem in the form of the formation of Schottky junctions.
In a recent study, which was published online on August 30, 2023 and will be published in January 2024 in volume 340 of the journal Applied Catalysis B: Environmental. They demonstrated an electrode that formed a Schottky junction at the interface of a metallic nickel tungsten nitride (Ni-W5N4). And a semiconducting n-type nickel-iron (oxy)hydroxyl (NiFeOOH) catalyst. The electrode was able to overcome the conductivity limit of the (hydroxyl) hydroxide and improved the water decomposition of the device.
Remarkably, the team linked a metal and a semiconductor, two materials with very different electronic behaviors. An interface of differing energies created, forming a Schottky junction. Dr. Seo explains, “Our study exploited this potential energy barrier present in the Schottky junction, accelerating the flow of electrons through the electrodes. This led to a significant increase in oxygen evolution reactivity. It accelerated the overall water decomposition process.” and emphasized the core mechanism behind their newly designed electrodes.
Electrocatalytic water decomposition
While performing electrocatalytic water decomposition, the team observed that the Ni-W5N4 alloy catalyzed the hydrogen evolution reaction. A current density of 10 mA/cm2 generated at a small overpotential (11 mV). In addition, the rectifying Schottky junction formed at the Ni-W5N4 | NiFeOOH interface counteracted the non-conducting layer produced by the (oxy)hydroxyl species. It exhibits a current density of 11 mA/cm2 at 181 mV overpotential under forward bias. Electrochemical analysis of the electrode showed that the improved catalytic activity can indeed attributed to the Schottky junction.
Finally, the researchers fabricated an electrolyzer for industrial seawater electrolysis using Schottky junction electrodes they designed. They found that the new device could run continuously for 10 days. It also showed excellent catalytic activity and durability during electrolysis. It exhibited an impressive current density of 100 mA/cm2 at an overpotential of only 230 mV.
Overall, the researchers believe that these findings could provide a sustainable strategy for hydrogen production, eventually replacing traditional methods that still rely on fossil fuels. As Dr. Seo concludes, “Freshwater and seawater are abundant and renewable sources of protons. Efficient water decomposition systems ensure that we can build sustainable production of zero-carbon hydrogen fuels that can help solve our current climate problems.”