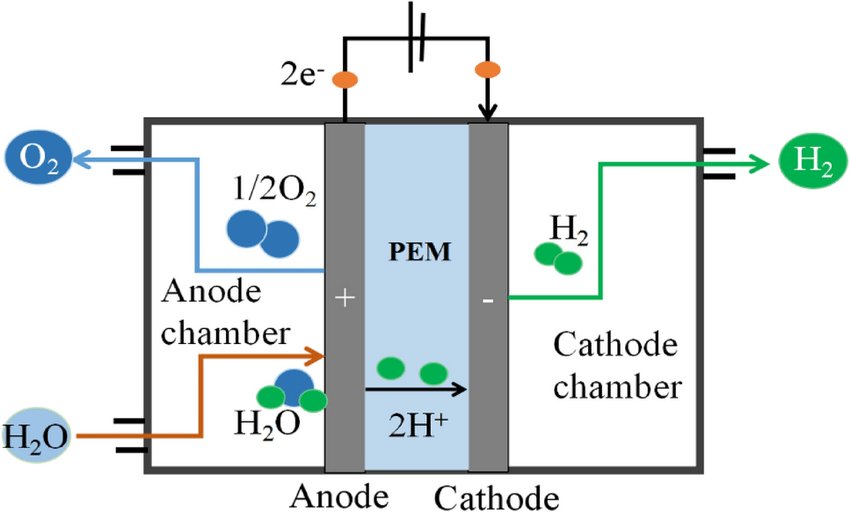
PEM hydrogen production and alkaline water electrolysis are two common methods for generating hydrogen. Both methods involve the use of an electrolyzer to split water into its component parts of hydrogen and oxygen. However, there are some significant differences between the two methods, including their efficiency, cost, and environmental impact.
What is PEM Hydrogen Production?
PEM (proton exchange membrane) electrolysis is a relatively new technology. It has gained popularity in recent years due to its high efficiency and low operating costs. In this method, the electrolyzer employs a proton exchange membrane to separate the anode and cathode compartments, thereby enabling a higher level of control over the reaction. This results in a higher efficiency rate, as less energy lost to heat and other byproducts.
Why choose PEM Hydrogen Production?
On the other hand, researchers and engineers have been using alkaline water electrolysis for many years. They making it a more established technology. In this method, an alkaline solution (usually potassium hydroxide) used as the electrolyte. It allows for a lower operating voltage and higher current density. However, this also means that the reaction is less efficient overall, as more energy lost to heat and other byproducts.
In terms of cost, PEM electrolysis is generally more expensive to install than alkaline water electrolysis due to the complexity of the technology. However, due to its higher efficiency and lower operating costs. It often proves to be more cost-effective in the long run. Alkaline water electrolysis, on the other hand, is generally cheaper to install but may be more expensive to operate over time due to its lower efficiency.
When it comes to environmental impact, both methods have their pros and cons. PEM electrolysis generally offers higher environmental friendliness due to its ability to reduce greenhouse gas emissions by virtue of its high efficiency and low operating costs. However, the production of the proton exchange membrane itself can be energy-intensive and may require the use of toxic chemicals.
Alkaline water electrolysis, on the other hand, produces hydrogen using a relatively simple and environmentally friendly process. However, the use of potassium hydroxide as the electrolyte can be problematic if not handled properly. As it can be corrosive and toxic.
In conclusion, both PEM hydrogen production and alkaline water electrolysis have their advantages and disadvantages. While PEM electrolysis is generally more efficient and cost-effective in the long run. It may be more expensive to install and may have a higher environmental impact during production. Alkaline water electrolysis, on the other hand. It is a more established technology that is cheaper to install but may be less efficient overall and has its own environmental concerns. Ultimately, the choice between the two methods will depend on a variety of factors, including cost, efficiency, and environmental impact.